Details of the Drug
General Information of Drug (ID: DMF9G7L)
| Drug Name |
Dibucaine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cincainum; Cinchocaine; Cinchocainum; Cincocaina; Cincocainio; Dermacaine; Dibucain; Dibucainum; Nupercainal; Nupercaine; Percamine; Sovcaine; Cinchocaine HCL; Cinchocaine hydrochloride; Cincocaina [DCIT]; Dibucaine Base; Dibucaine [USP]; Alpha-Butyloxycinchoninic acid diethylethylenediamide; Cincain (TN); Cinchocaine (INN);Cinchocainum [INN-Latin]; Cincocainio [INN-Spanish]; Dibucaine (USP); Nupercainal (TN); Nupercainal (VAN); Nupercaine (TN); Sovcaine (TN); Alpha-Butyloxycinchonic acid-gamma-diethylethylenediamine; N-[2-(Diethylamino)ethyl]-2-butoxycinchoninamide; N-(2-(Diethylamino)ethyl)-2-butoxycinchoninamide; QUINOLINE,2-BUTOXY,4-CARBOXY,(N-TRIETHYLAMINO) AMIDE CINCHOCAIN; 2-Butoxy-N-(2-(diethylamino)ethyl)cinchoninamide; 2-Butoxy-N-(beta-diethylaminoethyl)cinchoninamide; 2-Butoxy-N-(beta.-diethylaminoethyl)cinchoninamide; 2-Butoxy-N-[2-(diethylamino)ethyl]-4-quinolinecarboxamide; 2-Butoxy-N-[2-(diethylamino)ethyl]cinchoninamide; 2-Butoxy-quinoline-4-carboxylic acid (2-diethylamino-ethyl)-amide; 2-Butoxyquinoline-4-carboxylic acid diethylaminoethylamide; 2-N-Butoxy-N-(2-diethylaminoethyl)cinchoninamide; 2-butoxy-N-(2-diethylaminoethyl)quinoline-4-carboxamide; 2-butoxy-N-(alpha-diethylaminoethyl)cinchoninamide; 2-butoxy-N-[2-(diethylamino)ethyl]quinoline-4-carboxamide
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Anesthetics
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
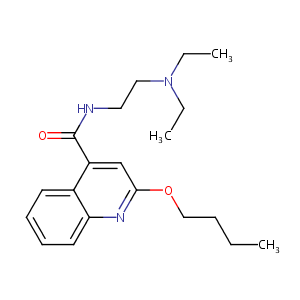 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 343.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.4 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Dibucaine (Comorbidity)
|
|||||||||||||||||||||||||||||
References
